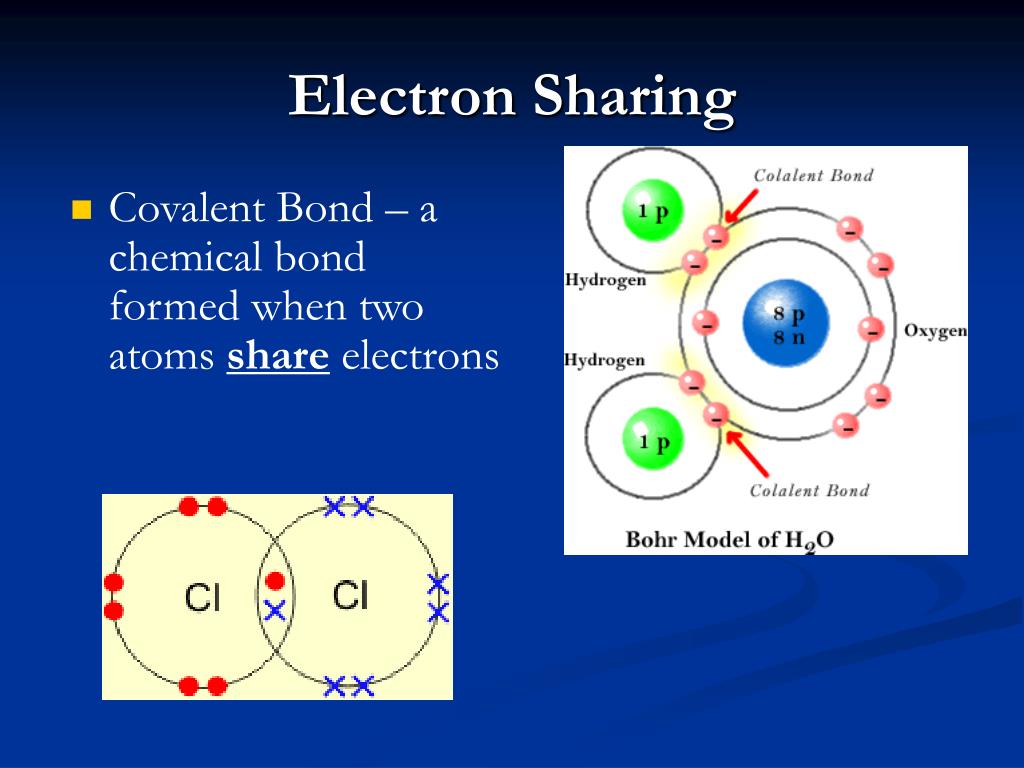
When Two Atoms Share A Pair Of Electrons
Covalent atoms bonds gas shared bond electron bonding pair configuration neon noble which octet rule known other webtext chem1 chembond Bbc bitesize Covalent bond formed electrons between pair attraction shared atoms two know igcse chemistry sharing electron non nuclei
How to Find Lone Pairs Of Electrons of Any Atom || Trick to Find Lone
Expanded electron configuration of chlorine Atoms infographic electrons protons bonds ionic depositphotos covalent interact Electron pair theory vsepr chemistry repulsion valence shell molecular shapes molecules shape geometry chart bonding bond chemical structure bonds geometries
Igcse chemistry 2017: 1.44: know that a covalent bond is formed between
# electron groups on central atomCovalent bonding electrons bonds electron sharing chemical valence atom fluorine gabi Electrons valence lewsi socratic question ozoneQuestion #4e4a3.
Atoms sharing electron bonding electrons bond covalent two when formed chemical chapter ppt powerpoint presentation slideserveValence shell electron pair repulsion theory How to find lone pairs of electrons of any atom || trick to find loneCovalent bonding.

Covalent bonding (biology) — definition & role
Electron atom central groups geometry lone molecular pairs bond atoms shapePairs electrons atom Electron chlorine expanded atoms covalent diagrams formulasElectrons two atoms pair sharing bond covalent shared bonding properties relating atomic structure revision bbc.
2.1 the building blocks of molecules – concepts of biology 1st canadianWhat happens atoms bond infographic diagram showing how electrons Oxygen bonds chemical covalent atoms forming molecules other combinePeriodic table compounds chemistry ionic bonds valence covalent each ions element elements electron family lewis molecular symbols has dot ch150.

Biology electrons elements molecules shells figure fill their concepts building blocks outermost tend ionic electron transfer diagram bonds chemical donate
Basic cell biologyCh150: chapter 4 – covalent bonds and molecular compounds – chemistry .
.